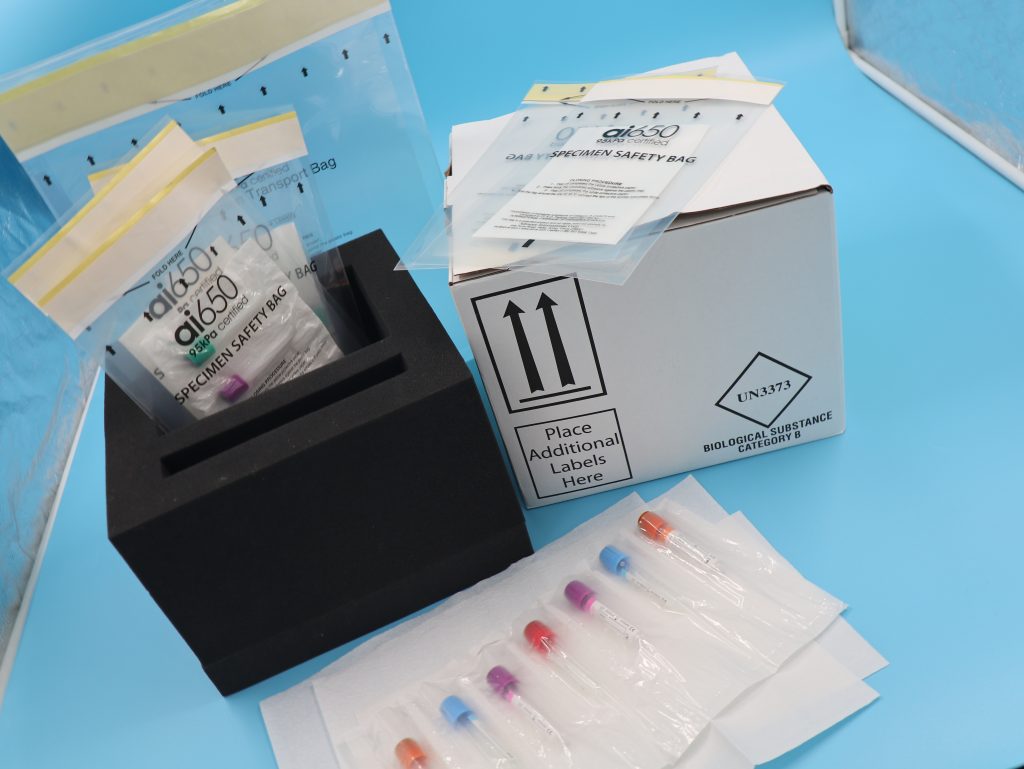
ai650 packing instantly absorb and solidify fluid waste and are non-toxic and are easy and ready to use premeasured pouches. Help reduce handling, shipping and compliance risks of diagnostic specimens, infectious substances and dangerous goods making both of these items very reliable and helpful when complying to the UN3373 regulations.
Infectious substances frequently include the following: Biological Substance, Biological Products, Patient Specimens, Medical Waste.

UN3373 is a DG shipment classification under IATA DGR -Infectious Substances. Infectious Substances are substances which are known or are reasonably expected to contain pathogens. Pathogens are defined as micro-organisms (including bacteria, viruses, parasites, fungi) and other agents such as prions, which can cause diseases in humans or animals. Infectious substances can be classified into two categories: A or B according to the level of infectiousness.
Category A is an infectious substance which is transported in a form that, when exposure to it occurs, is capable of causing permanent disability, a life-threatening or fatal disease in otherwise healthy humans or animals. Any biological infectious substance which does not meet the criteria for inclusion in Category A will be assigned to Infectious Substances Category B with identification number UN 3373, unless the source patient or animal has or may have a serious human or animal disease from a Risk Group 4 pathogen, in which case it must be classed as Division 6.2, described as an infectious substance, and assigned to UN 2814 or UN 2900, as appropriate.

Any packaging for biological substances must include three components, “A primary receptacle: the tube, vial or other container typically made of glass or rigid plastic (including the stopper, cap or other closure elements) that is in direct contact with the specimen. A secondary packaging (including cushioning and other materials) that fully encapsulates the primary receptacle. An outer packaging for shipping or transit. Components must meet specific requirements, including being capable of passing specific test procedures based on receptacle or packaging type. In addition, compliance with the regulations is based, in part, on overall performance; so there can be no substitutions of a component from one manufacturer with a similar – but untested – component from another manufacturer”(UN3773 Medical Packaging).